Life-saving TB drug is now cheaper in South Africa
Johnson & Johnson has agreed to drop the price of bedaquiline to the South African government by more than 40%
The South African government and pharmaceutical company Johnson & Johnson (J&J) have agreed to a lower price for bedaquiline, a medicine used to treat drug-resistant tuberculosis (DR-TB) in South Africa.
This comes off the back of mounting pressure from activists and amid an ongoing investigation by the Competition Commission, looking into J&J’s pricing of the drug.
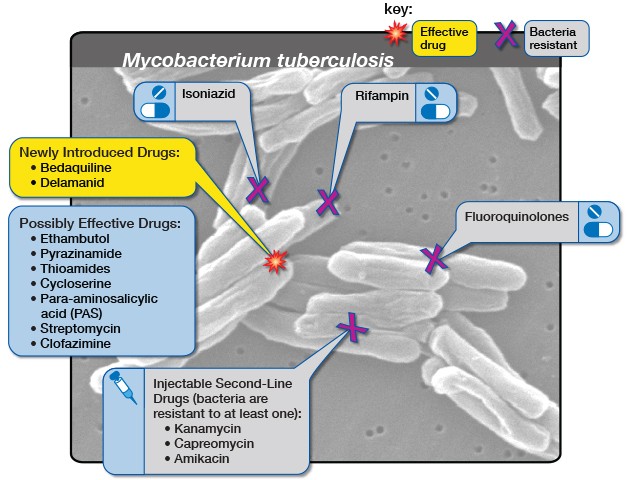
An estimated 14,000 people in South Africa fell ill with DR-TB in 2019. Bedaquiline is one of the main drugs used to treat DR-TB. Before bedaquiline became available, treatment for DR-TB would consist of up to two years of injections with serious side effects. The bedaquiline-containing regimen has no injectables, far fewer side effects and is typically six months.
Bedaquiline has been provided by the South African government since 2018.
In July, J&J agreed to sell bedaquiline to lower and middle-income countries through the Stop TB Partnership’s Global Drug Facility for $130 (R2,470) per six-month regime, but South Africa does not make use of this facility due to national procurement policies.
Instead, about the same time that J&J made this announcement, the National Health Department agreed to pay J&J R5,500 for the drug.
The Competition Commission announced in September that it will be investigating Johnson & Johnson’s pricing of the drug. The commission assisted the Department of Health in renegotiating the price, says department spokesperson Foster Mohale.
This week the department sent out a circular indicating that it will be paying R3,148 for bedaquiline.
Bedaquiline is prescribed to 7,000 to 8,000 people a year, Mohale told GroundUp. Mohale says the new price amounts to a 40% saving on bedaquiline for the next two years.
Candice Sehome, Access Campaign Advocacy Advisor for Medicines Sans Frontiere (MSF), told GroundUp that the “momentous” cost saving is a “big achievement”. Sehome says it is a sign that the global campaign to ensure accessible and affordable treatment for TB is yielding results.
MSF has estimated that bedaquiline could be manufactured and sold for profit for as little as $102 (R1,940).
Fatima Hassan, director of the Health Justice Initiative, says that while the price drop is a victory, it is important to ensure that this does not happen again.
“The significant price reduction emphasises why price scrutiny is significant,” Hassan told GroundUp.
Alleged “evergreening”
J&J’s patent for bedaquiline expired in July 2023, but J&J had already applied for a new patent for a slightly different version of bedaquiline, which was granted. This meant their patent protection continued in South Africa after the original patent expired.
ALSO READ: South African men are much more likely to die from TB than women – here’s why
This amounts to “evergreening”, says Hassan. Evergreening, as explained in this article in The Conversation, “is achieved by seeking extra patents on variations of the original drug – new forms of release, new dosages, new combinations or variations, or new forms”.
The Competition Commission will be looking into J&J’s alleged “evergreening” as part of its investigation.
After making its agreement with the Global Drug Facility, J&J has announced it will not be enforcing the new patent – a move that will allow generic versions of the product to enter the market and further lower the price.
GroundUp sent questions to J&J but received no response.
