Never-before-seen strain of ringworm discovered in KZN
Trichophyton indotineae,a never-before-seen strain of ringworm has been discovered in KwaZulu-Natal. Here is everything you should know.

Never-before-seen strain of ringworm has been discovered in KwaZulu-Natal.
NEVER-BEFORE-SEEN STRAIN OF RINGWORM DISCOVERED IN KZN
The National Institute for Communicable Diseases (NICD) confirmed a positive diagnosis was recorded in a patient in the Public Health Surveillance Bulletin.
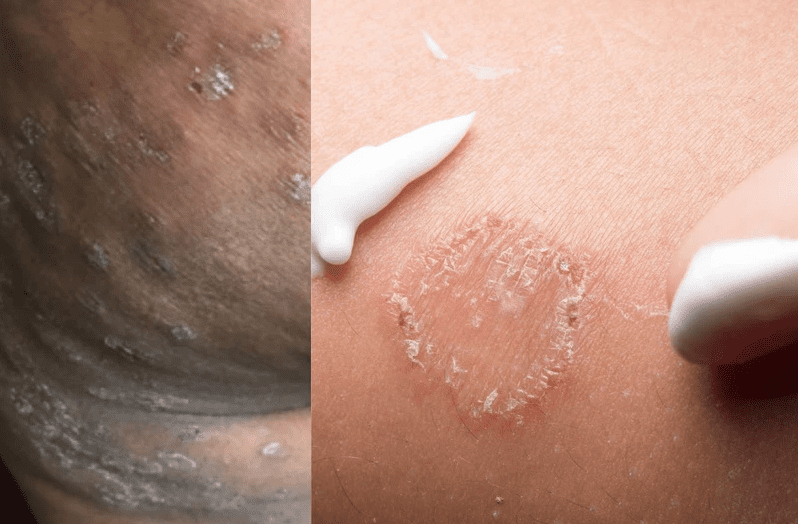
According to the NICD, ringworm (also known as tinea or dermatophytosis) is a common and often highly contagious fungal infection that involves the superficial layers of the skin, hair, and nails.
THE CASE WAS CONFIRMED IN KWAZULU-NATAL
“This is the first description of such a case in Africa, and the lack of travel history in the case patient indicates that community transmission is probably occurring in South Africa.” NICD said.
ALSO READ: Here is WHY Nesquik is DISCONTINUED in South Africa?
It furthermore said ringworm infections of the scalp or nails and extensive skin infections need a prolonged course of oral antifungal treatment, rather than topical treatment.
A never-before-seen strain of ringworm has been discovered in KZN, and it’s defying all treatments! This marks the FIRST instance in Africa, hinting at possible local spread. Uncover the alarming truth behind this skin infection causing chaos here: https://t.co/njLQiuLiMX pic.twitter.com/qqcuZCQoVO
— Public Health Bulletin South Africa (@TheBulletin_SA) December 20, 2023
RINGWORM IS A COMMON AND OFTEN HIGHLY CONTAGIOUS FUNGAL INFECTION
“Trichophyton indotineae, also referred to as Trichophyton mentagrophytes genotype VIII, is a recently-described dermatophyte mould that causes extensive and difficult-to-treat infections. Many strains of this particular fungus have genetic mutations that make it resistant to systemic antifungal medicines such as terbinafine.”
ALSO READ: KFC is a Christmas tradition in Japan
SAHPRA URGENTLY RECALLED A PRODUCT
Meanwhile, the South African Health Products Regulatory Authority (SAHPRA) recalled a product Lubri-A (Sterile Lubricating Jelly).
It said they are aware of the product Lubri-A (Sterile Lubricating Jelly) manufactured by Electro-Spyres, classified as Class B medical device and currently being distributed nationwide.
MULTIPLE COMPLAINTS HAVE BEEN RECEIVED ACROSS SA
“SAHPRA has been informed of multiple complaints received from health institutions, both public and private, across the country.
“The complaints are a result of a number of patients who became ill due to developing a fungal infection caused by exposure to the fungal species, Wickerhamomyces anomalus (previously Candida pelliculosa) associated with the use of Lubri-A (Sterile Lubricating Jelly).” – SAHPRA
Lubri-A is available in two presentations, the 2.5 g sachets and 50 g tubes.
THERE ARE MULTIPLE CONTAMINATED BATCHES
SAHPRA furthermore said considering the wide usage of the product for lubricating purposes in medical and surgical procedures, the Regulator has taken a decision to urgently recall this product from the market as there are multiple contaminated batches, with the potential to cause serious and widespread nosocomial infections.
“SAHPRA is alerting healthcare professionals and the public to discontinue the use of the product, remove it from their inventory and return it through their normal distribution channel(s) with immediate effect.
“As the source of the contamination of the product is still under investigation and not confirmed, all batches of Lubri-A are being recalled. Future manufacture and distribution of the product will be subject to review and authorisation by SAHPRA.” – SAHPRA
